September 5, 2024
Medications En Route To Deal With Obesity Epidemic
Tesofensine, A Novel Antiobesity Medication, Silences Gabaergic Hypothalamic Neurons Pmc In the USA and Europe, orlistat, naltrexone/bupropion, liraglutide 3 mg and, most just recently, semaglutide 2.4 mg are registered and advertised. On top of that, in the USA, phentermine/topiramate is even available for lasting use40. The stomach-derived peptide hormone ghrelin gets to the hypothalamus via the median prominence and promotes homeostatic food consumption through activation of NPY/AgRP neurons245, while promoting hedonic consuming through activation of dopaminergic neurons in the forward tegmental area302. To trigger its receptor, ghrelin needs N-octanoylation (acylation) at its serine 3 residue, and as dietary lipids are used for ghrelin acylation, this suggests that ghrelin might also work as a nutrient sensor that notifies the brain about incoming nutrients245. Research addition and exemption criteria for a randomized medical trial of Tesomet for hypopituitary individuals with hypothalamic weight problems.Excessive Weight
In a similar capillary, the dental cannabinoid receptor 1 (CB1) villain, rimonabant, was withdrawn in 2008 after just two years of regulatory authorization in Europe for monitoring of weight problems [30; Table 1] Regardless of encouraging rimonabant-induced cravings reductions, manifesting in substantial weight loss in human beings, the incident of serious cognitive negative impacts such as depression eventually brought about its withdrawal [30] Nonetheless, passion in inflection of the endocannabinoid system to manage obesity is still of substantial rate of interest, provided safer representatives with similar effectiveness can be discovered. Indeed, the future below may well depend on the advancement of selective cannabinoid receptor 2 (CB2) agonists, which have been demonstrated to reduce weight gain in the preclinical setup [31; Table 1] Nevertheless, it is essential to keep in mind that this reasonably current exploration of non-immune cell CB2 receptor actions mean considerable further work is required to completely confirm the effectiveness and security of this approach. " It is difficult to overlook this area," states Adam Cuttler, taking care of supervisor and elderly biotech analyst at Canaccord Adams. We can assist you attain your weight management objectives in 4Ever Young in Des Moines, IA, using tesofensine peptide, a life-altering, weight-loss medication. As opposed to a "one-size-fits-all" strategy, our patient-centered method gives them with a personalized treatment strategy customized to their certain requirements. Tesofensine is a serotonin-- noradrenaline-- dopamine reuptake inhibitor from the phenyltropane family members of drugs.Is tesofensine approved by the FDA?
The FDA gave orphan medicine classification for fixed-dose mix of tesofensine and metoprolol in PWS in March 2021 and hypothalamic excessive weight in July 2021. Tesofensine is a centrally acting monoamine reuptake inhibitor that obstructs the presynaptic reuptake of dopamine, serotonin, and noradrenaline.
- Finally, Tesomet at a dose of 0.5 mg tesofensine and 50 mg metoprolol was well-tolerated, did not impact heart price or high blood pressure, and caused a continual progressive weight reduction in adults with hypothalamic weight problems.
- Upon emerging reports of suicidal ideation and severe anxiety, the FDA denied its registration in 2007 (ref.334).
- Say goodbye to the restrictions of time and embrace a future loaded with vigor, self-confidence, and the flexibility to enjoy your age to the fullest.
- After demonstrating the anorexigenic impacts of tesofensine in lean Vgat-ChR2 mice, we aimed to duplicate our findings in obese Vgat-IRES-cre mice.
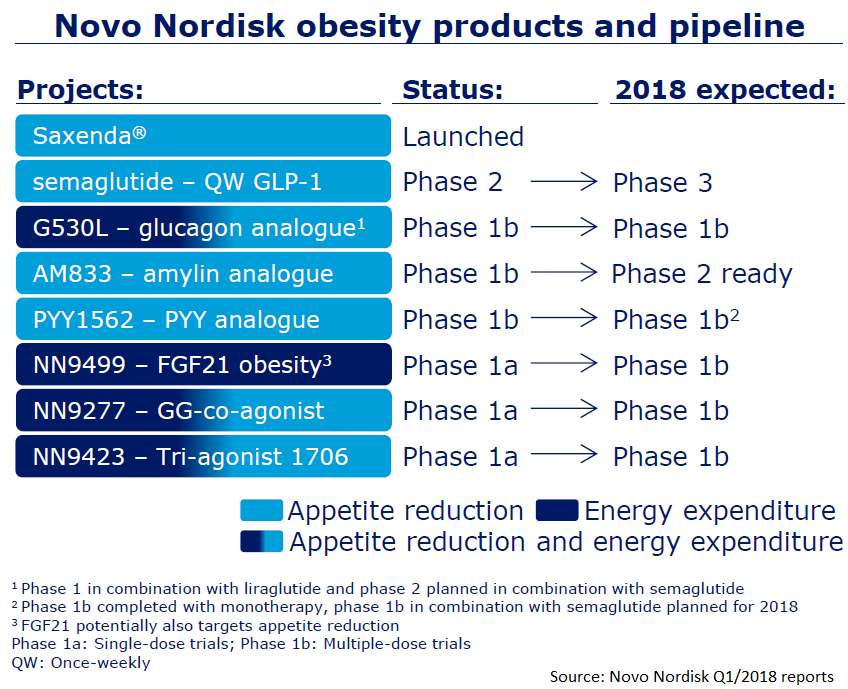
Drugs Registered For Obesity Treatment
Dopaminergic damaging drug reactions such as dyskinesias and stomach system and neuropsychiatric signs tended to be more constant in the groups getting greater tesofensine does. A greater percentage of individuals reacted with a minimum of 20% (array, 26% -40%) enhancement in UPDRS subscale II https://s3.us-east-1.amazonaws.com/pharma-regulations/clinical-trials/product-sustainability/tesofensine-an-unique-antiobesity-medication.html plus subscale III complete score in all the tesofensine arms of the trial compared to placebo (14%) (Table 3). A greater percentage of individuals responded with at the very least 20% improvement in off time in the 3 highest-dosage tesofensine therapy groups than in the sugar pill team. The distinction relative to placebo was statistically substantial only in the team obtaining tesofensine, 1 mg. Improvements relative to sugar pill in on time without bothersome dyskinesia were observed only in the group getting tesofensine, 0.25 mg. Patients in the groups getting tesofensine, 0.25 and 1 mg, seasoned increases know time with frustrating dyskinesia. Adjustments in satiation and food desires were not substantially different between teams (Table 7). 3 patients experienced severe negative occasions (SAEs); 2 randomized to Tesomet and one to placebo. In the Tesomet group, one individual created anxiety pertaining to Tesomet and the various other had recurrence of craniopharyngioma with succeeding post-procedural difficulties to surgery unconnected to Tesomet. In total amount, 64 damaging occasions (AE) were tape-recorded in 12 (86%) people randomized to Tesomet. Not every person who battles with weight encounters the exact same obstacles or needs the very same option. That is why our company believe in encouraging our individuals to not just lost excess weight but also to accomplish lasting, long-term outcomes. We satisfaction ourselves on our cutting-edge and individualized approach to weight loss, and our application of advanced treatments like Tesofensine and semaglutide treatment exemplifies our dedication to delivering phenomenal outcomes. 
Social Links