September 5, 2024
Tesofensine Discover The Scientific Research & Experts
Repurposed Representative Reveals Weight-loss Possible Nature Evaluates Drug Discovery The exact timeline might depend on factors such as individual metabolic rate, adherence to a prescribed diet regimen and exercise program, and the particular dosage of tesofensine being used. The concentration increased in a log-linear connection with the dose administered (Figure 2). Blood samples for pharmacokinetic and research laboratory evaluations were taken at baseline and at weeks 4, 6, 8, 10, and 14. Plasma focus of tesofensine were evaluated making use of a completely confirmed high-performance fluid chromatography tandem mass spectrometry approach at Boehringer Ingelheim, Biberach, Germany.Is Tesofensine A Maoi?
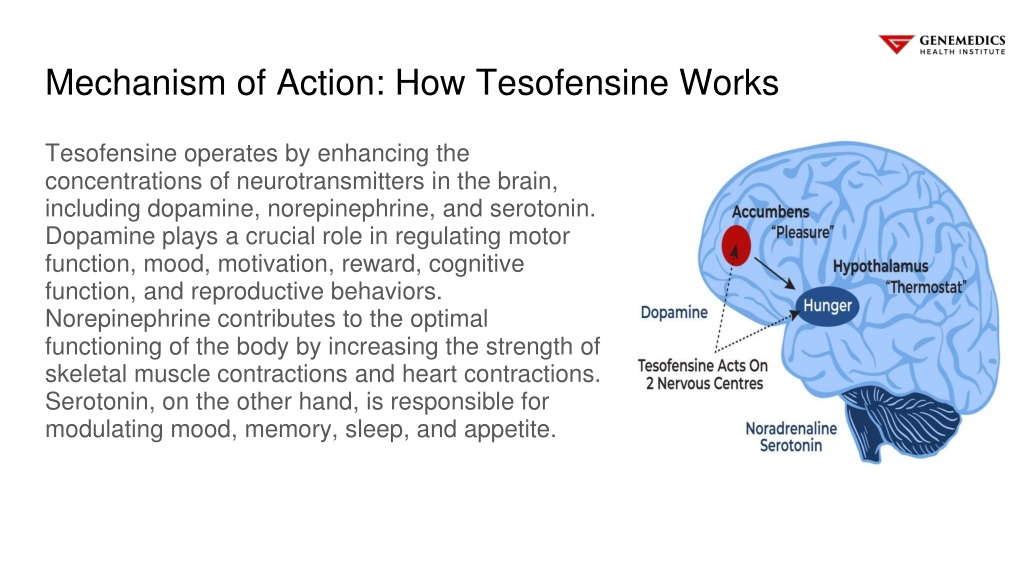
For people with emotional or mental conditions that take antipsychotics or antidepressants, caution is called for owing to the possibility for drug communications and enhanced danger of seizures [33] It has been proposed that tesofensine has a crucial dopaminergic element [3, 4, 42] Hence, the electric motor effects of tesofensine were compared versus phentermine, a characteristic dopamine-acting cravings suppressant. Our research study group lately reported that head weaving stereotypy is a common side effect of many appetite suppressants, particularly those acting to boost DA efflux, such as phentermine [15, 25] For that reason, we characterized the tesofensine-induced stereotypy results compared to phentermine, an amphetamine congener that served as a positive control. Experience Fleming Island's remarkable clinical weight management program, integrating comprehensive health analyses, individualized way of life alterations, medications, and high-grade supplements for transformative results. Uncover the efficiency of our remarkable clinical weight loss program readily available at our popular facility in Fleming Island. Immerse yourself in a customized technique that does not rely on surgical procedure or diet tablets. Our committed physicians will adeptly navigate evidence-based techniques that target the underlying causes of weight problems and weight gain. Presently, there are 3 classifications of anti-obesity medicines, consisting of (1) central nerves modifiers, (2) endocannabinoid preventions and (3) fat absorption inhibitors [4] Prior to medical licencing and commercialization, medicines should satisfy the guidelines by medication enforcement firms such as Health and wellness Canada, the American Fda (FDA) and the European Medicines Agency (EMA).Presently Authorized Long-term Treatments For Obesity
The federal government and the AMA appear prepared to accept the difficulty of lowering excessive weight. As demand for medicines rises, pharmaceutical and biotechnology firms will most certainly respond with new therapeutic entities to deal with obese and obese people. Until 2012, the US Food and Drug Administration (FDA) had not approved a medication to treat excessive weight since orlistat won authorization 13 years ago.2 Security issues had actually limited the number of medications https://us-southeast-1.linodeobjects.com/pharma-tech/Pharmacy-benefit-managers/product-strategy/tesofensine-an.html offered to deal with weight problems, and safety remains to continue to be a concern. Lorcaserin, marketed as Belviq by Sector Pharmaceuticals, and phentermine/topiramate expanded launch, marketed as Qsymia by Vivus Inc, were both accepted in 2012 to treat obesity alongside a reduced-calorie diet and exercise. Pharmacotherapy for obesity is suggested for individuals with a body mass index (BMI) ≥ 30 kg/m2 or 27-- 29.9 kg/m2 with a comorbid condition that boosts cardiometabolic threat [10]- Pramlintide is an amylin artificial analog that is hypothesized to affect amylin receptor activation in order to generate a satiating result, minimize food consumption and manage short-term energy homeostasis [10, 63, 64]
- Our clinically monitored fat burning program includes oral tesofensine peptide and the assistance of a group of specialists in Fort Lauderdale that determine the patient's weight loss by the number of pounds lost, their metabolic process, and body structure.
- However, benefits can start showing up within the initial couple of weeks and remain to boost with continuous therapy.
- In July of 2012, PHN/TPM (previously Qnexa ® [Vivus, Inc.] was accepted as a schedule IV drug under the brand, Qsymia ™.
- In addition, the management of psilocybin using IP injection may be expected to cause higher bioavailability than by dental intake.
Just how much weight can you shed on tesofensine?
For those taking the most affordable dose of 0.25 mg, average weight reduction was 6.5%, those taking the medium dose of 0.5% lost 11.2% and those taking the highest dosage of 1 mg shed 12.6%. In the two highest dosage groups, the treatment resulted in a 4 point decrease in BMI within of 24 weeks.

Social Links