August 16, 2024
Body Safety Compound-157 Improves Alkali-burn Wound Healing In Viv Dddt
Is Bpc 157 A Potential Wonder For Increasing Injury Healing And Restoring Peak Performance? This point was lately verified in a large research by Xu and partners (Xu et al., 2020). In this context, also for sensible objectives, supplying that the therapeutic results represent themselves, we supply an excellent background for more application of BPC 157 as a treatment. To reverse abdominal area disorder as a several occlusion disorder calamity, we enhanced the feature of the venous system with the secure gastric pentadecapeptide BPC 157. Therefore, by fixing and making up for harmed features, the turnaround of the chain of unsafe effects of high intra-abdominal pressure can be accomplished and stomach area syndrome recovery can happen. Thus, the beneficial searchings for in rats with significantly boosted intra-abdominal stress given the steady stomach pentadecapeptide BPC 157 (for review, see Sikiric et al., 2018) most likely occurred due to the effect on compressed crucial vessel tributaries, both arterial and venous, peripherally and centrally. The azygos blood vessel path was completely triggered in BPC 157-treated rats (and consequently supplied extra direct blood flow delivery), while it was collapsed in control saline-treated rats with intra-abdominal hypertension.Medical Examinations
In conjunction with blood vessel feature, we at least have toconsider leak of fluid/proteins/plasma, leading to edema/exudate development as well as thrombogenesis. In this element, we have neoangiogenesis resulting in pathological vascularization, vascular invasionresulting in launch of metastatic cells and the sensation of homing leading to development of second tumors-- metastases. BPC-157 is a peptide that has actually been shown to be efficient in reducing joint pain, improving joint mobility, enhancing recuperation from injuries, recovery skin burns, and musculotendinous injuries.Bpc 157 Peptide Bpc 157 Review, Side Effects, Dosage, Cycles, Before And After Results - Outlook India
Bpc 157 Peptide Bpc 157 Review, Side Effects, Dosage, Cycles, Before And After Results.
Posted: Tue, 08 Aug 2023 07:00:00 GMT [source]

Illuminating The Peptide's Device Of Activity Within Systems
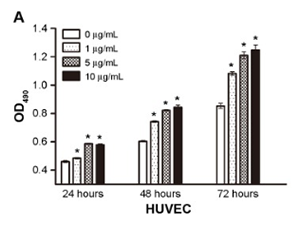
In the 2nd protocol, HUVECs (4 × 104 cells per well) in full media were concurrently seeded with DMSO or BPC-157 (1 μg/ mL, 5 μg/ mL, and 10 μg/ mL) in matrigel-coated plates. The encased networks of tubes were photographed 12 hours later utilizing Canon PowerShot A640 video camera on Zeiss upside down microscopic lense with × 100 magnifying. The setting of the cells in the cell cycle was figured out by circulation cytometric evaluation of the DNA material using propidium iodide. The cells were gathered after treatment, cleaned two times with cold phosphate-buffered saline, and treated with 1 mL of cold citrate buffer (0.24 M sucrose, 40 mM sodium citrate, pH 7.6). Ultimately, 0.4 mL of a PI staining/lysis remedy (0.5% NP-40, 0.5 mM ethylenediaminetetraacetic acid [EDTA] and 50 μL of RNase A (10 mg/mL in Tris-- EDTA buffer, pH 8.0) remedy were included.Examining Its Regenerative Effects On Tissues
This result recommends that BPC 157-treated rats show continual improvement in electric motor function also prior to tissue recovery, as observed by microscopy evaluation. The resolution of spasticity by day 15 (Fig. 2) suggests that BPC 157 management protects against the chain of occasions after spinal cord injury that is moderated by the loss of neighborhood segmental restraint and/or by an increased sensory afferent drive that results in the worsening of α-motoneuron activity [66] These findings validate the variety of big myelinated axons in the caudal nerve and the reduced MUP in the tail muscle. Therefore, specific theoretical assistance in rats with high intra-abdominal pressures is provided by intestinal tract failure, hemorrhagic lesions in the stomach, transmural hyperemia of the whole intestinal system, tummy, duodenum, and tiny and large digestive tract wall surface. The reduction of villi in the digestive tract mucosa and crypt decrease with focal denudation of surface epithelia and dilatation of the huge bowel show vascular failing (Chan et al., 2014). The other way around, the stabilized portal and caval stress and aortal pressure as a cause-consequence are convincing proof of the operating "bypassing key" (i.e., the azygos vein).- It docks with precision, launching a domino effect that resounds through signaling paths integral to cells repair service and regrowth.
- Lung parenchyma with significant blockage and big areas of intra-alveolar hemorrhage in control rats.
- Rats were laparatomized prior to sacrifice for the matching presentation of the outer vessels (azygos blood vessel, exceptional mesenteric vein, portal vein, substandard caval capillary, and abdominal aorta).
- These beneficial effects include the counteractions of distressing brain injury and extreme encephalopathies after NSAID overdose, insulin overdose, magnesium overdose, and direct exposure to the neurotoxin cuprizone in a rat design of multiple sclerosis [33,34,35,36,37,38,39,40,41]
- In the future, we will certainly perform medical trials for taking a look at BPC157 for the therapy of extreme injury and burns.
- In the design control group, the granulation tissues created were hypocellular and covered by a thin premature epithelium.
Is BPC 157 lawful in Europe?
The PUBCHEM ID is CID 9941957. The peptide is banned by the World Anti-Doping Firm in 2022 under the S0 group of non-exempt compounds.
Social Links