September 5, 2024
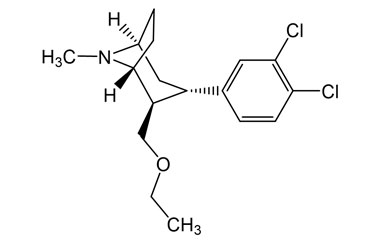
Tesofensine, An Unique Antiobesity Medicine, Silences Gabaergic Hypothalamic Neurons Pmc
An Around The World Annual Study Of Brand-new Data In Unfavorable Medicine Reactions
One female person display stopped working but was randomized in error and obtained a solitary dosage of Tesomet but completed no post-dose assessments. Hypopituitarism was pharmacologically taken care of in all however three women individuals randomized to placebo who chose not to have their hypogonadism or development hormonal agent shortage substituted. Ultimately, as private treatment actions to anti-obesity medicines vary, the suitable classification of individual groups will be the first step toward customized medicine and the stipulation of better drug selection and enhanced treatment algorithms. Additionally, pharmacogenetic and mechanistic research studies to verify the results of medicines on feeding behavior and reward processing would allow the further characterization of good responders to various anti-obesity drugs [77] Although liraglutide has no result at a reduced dosage, at a high dose, state of mind conditions aggravate slightly.What are the threats of taking tesofensine?
Tesofensine 0.25 mg, 0.5 mg, and 1.0 mg and diet plan caused a mean weight-loss of 4.5% (0.87 ), 9.2% (0.91 ), and 10.6% (0.84 ), specifically, greater than diet plan and placebo (p<
Gastrointestinal Conditions
- There has been substantial passion in this investigational medicine for weight decrease as an accessory to energy limitation.
- A similar end result caused making use of anti-ghrelin Spiegelmers created at NOXXON Pharma that only moderately boosted metabolism in preclinical research studies, without effect on food intake after 8 days of treatment246.
- This action of buproprion was gone along with by a rise in dopamine degrees and in the phosphorylated type of the dopamine and cyclic-AMP associated phosphoprotein 32 kDaltons (pDARPP-32) in the nucleus accumbens core (Randall et al., 2014).
- Due to the function of DA in the benefit mind system, it can cause manic episodes in bipolar disorders.
Lose Weight Safely And Efficiently With Tesofensine Peptide In Des Moines, Ia
" Concerns concerning cravings suppressants and the possible side-effects of tachycardia and boosted high blood pressure have actually been really off-putting," describes Shahred Taheri, a metabolic rate specialist at the Henry Wellcome Laboratories for Integrative Neuroscience and Endocrinology in Bristol, UK. More clients in the pooled tesofensine treatment teams (81.5%) than in the sugar pill group (73.5%) experienced unfavorable events (Table 4). Clients in the tesofensine therapy groups experienced a greater rate of nerve system problems (dyskinesia and headache), gastrointestinal system conditions (nausea or vomiting and irregular bowel movements), and psychiatric conditions (hallucinations and sleep problems). The incidences of serious unfavorable events were 20.4% in the placebo group and 16.6% in the pooled tesofensine treatment groups. The null theory was that there was no distinction in between patients treated with sugar pill and individuals treated with tesofensine at any kind of dosage. The analytical tests made use of for the regression coefficient and for the comparison of tesofensine and sugar pill were 1-sided 2-sample examinations at a 5% relevance degree. No statistical adjustment for having 2 coprimary outcomes or multiple contrasts was made. Analytical analyses were considered descriptive just because of the exploratory layout of the pilot trial.Social Links